BASIC LAW OF CHEMISTRY
a. Law of Conservation of Mass (Lavoisier's Law) "The mass of substances before and after the reaction is the same".Example:S + O 2 → SO 22 gr 32 gr 64 grb. Fixed Comparative Law (Law Proust) "Comparison of the mass of each element in the compound is still"Example:H 2 O → mass of H: mass of O2: 16 = 1: 8c. Multiple Comparative Law (Law Dalton) "If these two elements can form two or more compounds, and the mass of
each one of the elements, the mass ratio of the second element as an
integer value and simple".Example:- Elements N and O to form compounds NO and NO 2- In NO compounds, mass mass N = O = 14: 16- In the compound NO 2, N = mass mass O = 14: 32- Comparison of the mass of N in NO and NO 2 at theO mass ratio = 16: 32 = 1: 2d. Ideal Gas Law For an ideal gas or a gas that is considered ideal apply the formula: P V = n R T Which are: P = pressure (atmospheric) V = volume (liters) n = mole = gram / Mr R = the gas constant (lt.atm / mol.K) T = temperature (Kelvin)
THE MOLES CONCEPT
a. In chemistry unit amount used is mole
b. one mole is the amount of substance containing 6.02 x 10 ^ 23 particles
Mole relationship with the number of particles
Particle number = mol x 6.02 x 10 ^ 23
moles = Number of particles / 6.02 x 10 ^ 23
Mole relationship with mass
For elements:
mole = mass / Ar
mole = mass x Ar
To compound:
mole = mass / Mr
mass = mole x Mr.
Mole relationship with Gas Volume
Each one mole of any gas ATP state has a volume: 24 liters.
The volume of gas = mole x 22.4
mole = Volume / 22.4
Relations moles, number of particles and gas relationship can be described in the form
diagram as follows:

PROBLEM SOLVING
1.
Sb2S3(s) + 3Fe(s)→2Sb(s) +3FeS(s)
If 3.87×1023 particles of Sb2S3(s) are reacted with excess Fe(s), what mass of FeS(s) is produced?
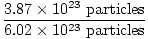 ×1 mole Sb2S3(s) = 0.643 moles Sb2S3(s) ×1 mole Sb2S3(s) = 0.643 moles Sb2S3(s) |
||||||
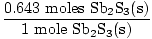 ×3 moles FeS(s) = 1.93 moles FeS(s) ×3 moles FeS(s) = 1.93 moles FeS(s) |
||||||
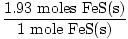 ×88 grams = 170 grams ×88 grams = 170 grams |
2. Given chemical reaction as follows:

If 71 grams of Cl 2 produced in the above reaction specify:
a) the mass of H2O is formed
b) the mass of KCl obtained
c) HCl required mass
d) mass necessary KCLO3
(Ar K = 39; Cl = 35.5; O = 16; H = 1)
Discussion =
From the data (Ar K = 39; Cl = 35.5; O = 6; H = 1) is obtained:
Mr KCLO3 = 122.5
Mr H2O = 18
Mr HCl = 36.5
Mr KCl = 74.5
Mr Cl 2 = 71
Cl2 produced as much as 71 grams means the number of moles of Cl 2 is:
mol = gram / Mr
mol = 71/71 = 1 mol.
so that
a) the mass of H2O is formed
Mr mass = moles x = 1 x 18 = 8 grams
b) the mass of KCl obtained
mass = mol x Mr = 1/3 x 74.5 = 24.83 grams
c) HCl required mass
mass = mol x Mr = 2 x 36.5 = 73 grams
d) mass necessary KCLO3
mass = mol x Mr = 1/3 x 122.5 = 40.83 grams
3. If 4.4 grams of propane is burned perfectly according to the reaction:
C3H8 (g) + O2 (g) → CO2 (g) + H2O (l) (yet similar). Find the volume of carbon dioxide gas produced at STP where (Ar C = 12; H = 1)
Discussion
The equilibrium reaction as follows:
C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O (l)
propane C3H8
Mr = 3⋅12 + 1⋅8 = 44
mol = gram / Mr
= 4.4 / 44
= 0.1 mol
Carbon dioxide (CO2) produced:
= (CO2 coefficient / C3H8 coefficient) C3H8 mole × 22.4 × liter
= (3/1) × 0.1 × 22.4 liters
= 6.72 liter
No comments:
Post a Comment