Science Blog
Wednesday, 15 June 2016
Friday, 18 March 2016
Hydrocarbons
DEFINITION
TYPES OF HYDROCARBON COMPOUNDS
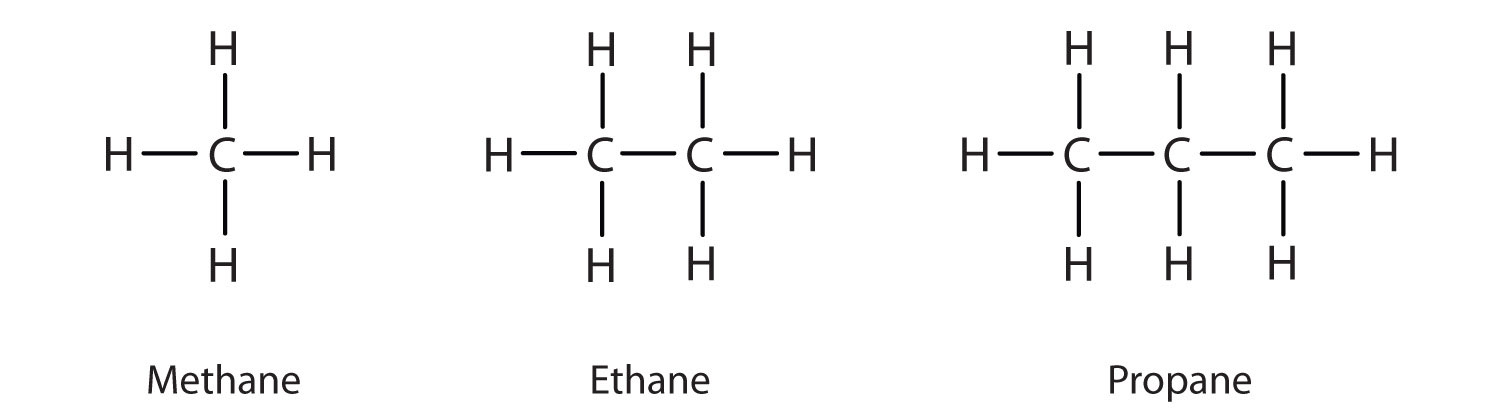
Hydrocarbons are organic compounds that made no more than carbon and hydrogen. It is possible to bind two or three formed between carbon atoms, and even to the structure formed as a ring.
TYPES OF HYDROCARBON COMPOUNDS
Alkanes
Alkanes are saturated hydrocarbons which means they are compounds with a single bond between atoms. Saturated and unsaturated hydrocarbons with hydrogen is the simplest. They are generally represented by the chemical formula CnH2n + 2 in the case of non-cyclic structure or straight-chain structure.
Molecular Shape
Electron Domain Theory
● The shape of molecules depending on the room arrangement bonding pair (B)
and a pair of free electrons central atom (F) in the molecule. can be explained
the electron pair repulsion theory valence shell or VSEPR theory (Valence
Shell Electron Pair Repultion)
● molecule covalent electron pair are both B and F.
Because the electron pair have like charges, then between electrons pair. Repulsion
repulsion (B - B)
● The existence of repulsion causes the atoms bonded
form a certain space structures of a molecule thus
molecular shape is influenced by the amount of F and the B held on
the central atom.
● The shape of the molecule was determined by an electron pair bond
Examples of CH4 molecule has four B
Molecular Shape Table
 |
| http://chemwiki.ucdavis.edu/@api/deki/files/57957/=CNX_Chem_07_06_molgeom.jpg?revision=1 |
Chemical Bonding
DEFINITION
The chemical bonding is a bond that forms between atoms or between molecules by means of:
One atom loses electrons, while the other atoms accept electron
The use of shared pair of electrons originating from each atom bonded
The use of shared pair of electrons originating from one atom bonded
The purpose of chemical bond formation is to achieve the stability of an element. The stability of the element occurs when an element follow the octet rule. Octet rule is the tendency of these elements to make the electron same configuration as the noble gases. The noble gases (VIII-A group) have as many as eight valence electrons (octet) or 2 (duplet, only the element Helium).
Thursday, 17 March 2016
Rate of Reaction
DEFINITION
Rate of reaction stating the number of chemical reactions that take place per unit time. The reaction rate stated molarity of the solute in the reaction generated each second reaction.
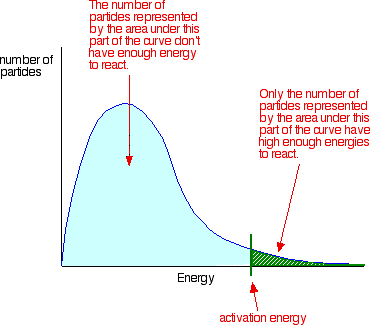
MAXWELL-BOLTZMANN DISTRIBUTION CURVE
Maxwell-Boltzmann distribution describes the speed of the particles in the gas and the particles do not constantly interact with one another, but move freely between short collisions.
Maxwell-Boltzmann distribution is usually regarded as the velocity distribution of molecules, but can also refer to the distribution of velocity, momentum, and the magnitude of the momentum of the molecule, which each will have a different distribution probability function, all of which related.
Rate of reaction stating the number of chemical reactions that take place per unit time. The reaction rate stated molarity of the solute in the reaction generated each second reaction.
MAXWELL-BOLTZMANN DISTRIBUTION CURVE
Maxwell-Boltzmann distribution describes the speed of the particles in the gas and the particles do not constantly interact with one another, but move freely between short collisions.
Maxwell-Boltzmann distribution is usually regarded as the velocity distribution of molecules, but can also refer to the distribution of velocity, momentum, and the magnitude of the momentum of the molecule, which each will have a different distribution probability function, all of which related.
Stoichiometry
BASIC LAW OF CHEMISTRY
a. Law of Conservation of Mass (Lavoisier's Law) "The mass of substances before and after the reaction is the same".Example:S + O 2 → SO 22 gr 32 gr 64 grb. Fixed Comparative Law (Law Proust) "Comparison of the mass of each element in the compound is still"Example:H 2 O → mass of H: mass of O2: 16 = 1: 8c. Multiple Comparative Law (Law Dalton) "If these two elements can form two or more compounds, and the mass of
each one of the elements, the mass ratio of the
Atoms and Elements
WHAT IS ATOM?
Atoms are the building blocks of all matter that exists. They are so small that we can not even see with our naked eyes. Atoms usually in the range of Angstrom.
After much experimentation, the atomic structure described in the 19th century. Atoms consist of a nucleus, which has a proton and a neutron. In addition to neutrons and positrons there are other small sub-atomic particles in the nucleus. And there are electrons circling the nucleus in the orbital.
Most of the atom is empty space.
Subscribe to:
Posts (Atom)